The Glenoid Labrum
Embryology |
Introduction
The improvement in clinical imaging and the development of shoulder arthroscopy over the last twenty to thirty years has allowed clinicians to diagnose and treat more subtle pathologies of the glenohumeral joint. Specifically injuries to the labrum have been more recognised during this period, as a source of symptoms and the optimal treatment of these lesions is evolving at a rapid pace. This review presents the current knowledge of the glenoid labrum.
Embryology
Synovial joints develop by the formation of a primitive anlage composed of the cartilaginous precursors to the individual bones, with a transverse band of flattened cells (interzone) separating these two sides of the joint. For the shoulder, the interzone within the anlage appears at 11-12 mm CRL (crown-rump length) (6 weeks) with a vascular periphery, whilst the centre remains avascular and begins to cavitate at around 22 mm CRL (8 weeks) (Gardner and Gray 1953) . The dehiscence of the two sides is said to be complete by 34 mm CRL (9 weeks)(Haines 1947b) . The fibrous capsule is evident at 16 mm CRL (7 weeks) (Haines 1947a) and the anlage of the labrum by 17 mm CRL (7 weeks) (Gardner and Gray 1953). By 21 mm CRL (8 weeks) the labrum can be seen to be deficient in the region deep to the coracoid process, in the rest of the circumference a few collagenous strands start to appear. At 27 mm CRL (8.5 weeks) the labrum is more pronounced posteriorly than anteriorly. At this stage it has been postulated that the anterior labrum deep to the subscapularis tendon has been formed from the synovial mesenchyme, as apposed to the labral anlage, as it does not join the cartilage of the glenoid (Gardner and Gray 1953).
More recent work (Aboul-Mahasen and Sadek 2002) has specifically looked at the progression of the labrum and other intra-articular structures from 9 weeks gestation onwards. At 9 weeks (30 mm CRL) the glenoid labrum, and biceps tendon can be visualised, with the biceps tendon inserting into the superior aspect of the labrum. At this stage the glenoid fossa and humeral head are poorly developed. Microscopic assessment shows the labrum as a primitive fibrous condensation on the margin of the glenoid fossa, with intermingling of both the biceps and triceps tendons. A fibrocartilagenous transition zone can be seen between the fibrous labrum and the hyaline articular cartilage.
By 12 weeks (60 mm CRL) the fossa has become pear-shaped with increasing concavity. The humeral head is now a hemi-sphere and the surgical neck can be identified. The labrum has thickened, except in the anterosuperior part, where it appears meniscus like. The superior, middle and inferior glenohumeral ligaments are beginning to appear as thickenings in the joint capsule.
At 16 weeks (120 mm CRL), the labrum has thickened again, the biceps appears as an extension of the superior labrum and the inferior ligament has a wide attachment to the anterior and inferior part of the labrum. Microscopically at 16 weeks the superior labrum has become more fibrous and vascular, whereas the posterior labrum appears more fibrocellular.
By 23 weeks (198 mm CRL) all the intra-articular structures had taken on the form that is seen in the adult shoulder, except the biceps tendon which still appeared cord shaped. The posterior labrum is now more fibrocartilagenous and the whole of the labrum is more vascular.
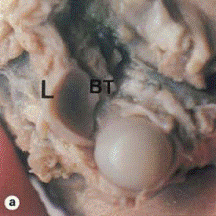
Figure 1. Posterior view of a 23 week gestational shoulder. Showing the thick circular ring of the glenoid labrum (L) and the biceps tendon inserting into it (BT) (Aboul-Mahasen and Sadek 2002).
At full term (40 weeks and 370 mm CRL) the labrum forms a well defined ring, deepening the concavity of the glenoid fossa. However, the thickness is still less anteriorly. The posterior labrum has become hyper-cellular with groups of chondrocytes, chondrobalsts and fibroblasts. The biceps tendon now has a flattened appearance. When compared to the adult shoulder, the joint cavity is relatively smaller with less synovial covering and folds.
It appears that even as early as 21 mm CRL (8 weeks) the anterosuperior part of the labrum is different to the rest of the labrum and may even be derived from different tissue. A further cadaveric study has also observed that an area of detachment of the labrum in the anterosuperior region was present in specimens over 22 weeks gestation (Fealy et al. 2000) and arthroscopic assessment of 20 foetuses found the anterosuperior labrum to be detached in 10% of the shoulders (Tena-Arregui et al. 2005).
This area is of particular anatomical debate in the adult, and these studies suggest that this difference is presence from a very early age.  top
top
Anatomy
The adult labrum is a circumferential fibrocartilagenous structure, variably attached to the glenoid rim, providing a site of attachment for the glenohumeral ligaments. The radial thickness, from its inner to outer circumference, varies from 2 mm inferiorly to 11 mm superiorly, and is between 5 mm and 9 mm deep, suggesting this fibrocartilagenous structure contributes to the glenoid depth by 30% to 50% (Howell and Galinat 1989; Lippitt and Matsen 1993). It has been described as having a triangular cross-section superiorly and as a more rounded elevation inferiorly (Cooper et al. 1992).
The anterosuperior region is the most inconsistent area of the labrum and at arthroscopy there may be a complete absence of any labral tissue in this region (1.5%), a sub-labral foramen (11.9%) (Rao et al. 2003) or a mobile labrum (26%) (Davidson and Rivenburgh 2004). However, anatomical dissection studies have suggested the incidence of a sub-labral recess may be as high as 74-85% (Harzmann et al. 2003; Waldt et al. 2006) with a sub-labral foramen in 11% (Waldt et al. 2006). A sub-labral foramen or hole being defined as a complete detachment of the labrum from the glenoid, as opposed to a sub-labral recess or sulcus, where the labrum is lifted off the glenoid at the articular surface, but there is still a deeper attachment. It is probable that the variation in this region is due to both the embryological development and confusion, or at least non-uniformity in nomenclature and recognition of these variables.
If this area has been derived from tissue other than the labral anlage, it is likely to have different mechanical properties. This may make it more susceptible to injury during further growth and could produce the wide range of variants seen in the adult shoulder. There is certainly an increasing prevalence of structural defects and tears with age (Prodromos et al. 1990; Pfahler et al. 2003; Clavert et al. 2005) and it has been hypothesised that detachment in this region may represent a secondary synovialised labral tear sustained in the second decade of life (De Palma et al. 1949; Prodromos et al. 1990). The labrum may therefore have a loose attachment, attachment to the middle or inferior glenohumeral ligament or no attachment at all (Cooper et al. 1992).
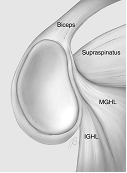
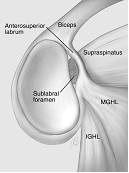
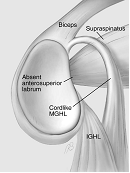
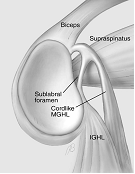
Figure 2. Common findings in the anterosuperior quadrant. 1) 'normal' anatomy, 2) Sublabral foramen, 3) Buford complex, 4) Sublabral foramen and cord-like middle GHL (Powell et al. 2004).
The Buford complex is an example of one of these variants whereby the anterosuperior labral tissue is absent and the middle glenohumeral ligament takes on a cord-like appearance (Buford et al. 1992; Williams et al. 1994). Inappropriate 'repair' of this 'lesion' can result in both pain and limitation of external rotation.
As well as the glenohumeral ligaments, the biceps tendon inserts into the labrum. The point of insertion is variable, but will attach at some point between the anterosuperior and posterosuperior quadrant (Pal et al. 1991; Vangsness, Jr. et al. 1994; Demondion et al. 2001; Tuoheti et al. 2005). The presence of the glenohumeral ligaments seems to be inconsistent (Steinbeck et al. 1998; Ide et al. 2004), but when present, the superior and middle glenohumeral ligament attach between the 12 and 1 o'clock position, whereas the anterior-inferior glenohumeral ligament has a much more variable insertion from between 1 and 5 'o'clock (Ide et al. 2004; Tuoheti et al. 2005) (The clock positions refer to a glenoid of a right shoulder, with the 12 o'clock position at the superior margin). A posterior superior glenohumeral ligament has also recently been described, although this does not arise directly form the labrum (Pouliart et al. 2007). The long head of triceps, according to some authors (Hertz et al. 1986; Huber and Putz 1997), takes a partial origin from the inferior labrum as well as the infraglenoid tubercle.
The labrum takes attachment to the underlying glenoid. This is classically described as tear-drop, or oval shaped (Anetzberger and Putz 1996) and has a small surface area when compared to that of the humeral head. It has a mean anterior-posterior width of 27 mm, and a superior-inferior height of 34-35 mm (Lippitt and Matsen 1993; McPherson et al. 1997). The articular surface diameter of the humeral head varies from 36-52mm (Boileau and Walch 1997). As well as a difference in surface area the radii of the hemi-ovoid humeral head and the concave glenoid differ significantly (Soslowsky et al. 1992). The radius of curvature of the humeral head is 23-24mm in anterior-posterior plane and 21-23mm in the superior-inferior plane (McPherson et al. 1997; Hertel et al. 2002), whereas the radius of the glenoid is 32mm in the anterior-posterior plane and 41mm in the superior-inferior plane (McPherson et al. 1997). The bony glenoid depth is 5mm in the superior-inferior plane, but only 3mm in the anterior-posterior plane (McPherson et al. 1997). In summary, the bony dimensions demonstrate a large well-rounded humeral head balancing on a much smaller, shallow, relatively flat glenoid with unequal radii in the two planes. This allows for a large range of movement, but does not produce a stable joint without substantial additional support by the soft tissues. These additional soft tissues are described as either passive or active stabilisers. The active stabilisers are the actuators that are also involved in moving the joint: the superficial muscles such as the deltoid, and the deep muscles of the rotator cuff. The static stabilisers include the capsule, gleno-humeral ligaments and the glenoid labrum.  top
top
Microscopy
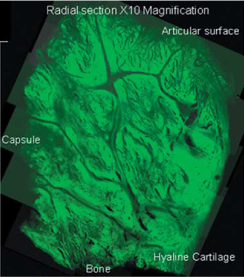
Figure 3. A radial section of the labrum at x 10 magnification (Hill et al. 2008).
Microscopy of the labrum has again caused confusion as to it's exact structure with some studies describing the labrum as a fibrocartilagenous structure containing dense cartilaginous fibrous tissue and chondrocytes (Prodromos et al. 1990; Nishida et al. 1996), whereas others describe the labrum as a fibrous structure with a small fibrocartilagenous transition zone between the hyaline cartilage and the fibrous labral tissue (Moseley and Overgaard 1962; Cooper et al. 1992; Huber and Putz 1997). This discrepancy may be due to both nomenclature and the exact position of the labrum from which the specimens were taken.
It has been demonstrated that the labrum is only vascular in its outer third and appears to obtain its blood supply from the periphery rather than the underlying bone. The superior and anterosuperior regions being less vascular than the inferior and posterior regions (Cooper et al. 1992). The suprascapular artery, circumflex scapular branch of the subscapular artery and the posterior circumflex humeral artery all contribute to the blood supply of the labrum (Cooper et al. 1992).
It is known to contain free nerve endings in its periphery (Vangsness, Jr. et al. 1995) and there is a suggestion that the labrum may be needed to allow full proprioceptive feedback (Machner et al. 1998), however as yet there is no conclusive evidence. Golgi's, Ruffini's, and Pacini's corpuscles as well as free nerve endings have been found in the glenohumeral ligaments, whereas the biceps tendon and labrum contain only free nerve endings (Guanche et al. 1999).
Both Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) have helped to demonstrate the collagen architecture of the labrum (Nishida et al. 1996; Hill et al. 2008). This has revealed three layers. The superficial layer with randomly orientated and loosely packed collagen fibres, thought to aid in lubrication. An intermediate layer and the core layer, which forms the bulk of the tissue. Within this core layer the collagen fibres are tightly packed and well orientated in a circumferential manner. However, there is vertical, oblique and interweaving fibres anchoring the labrum to the underlying bone. There is connection between the labrum and the capsule, GHL's and biceps tendon with intermingling of fibres (Nishida et al. 1996).  top
top


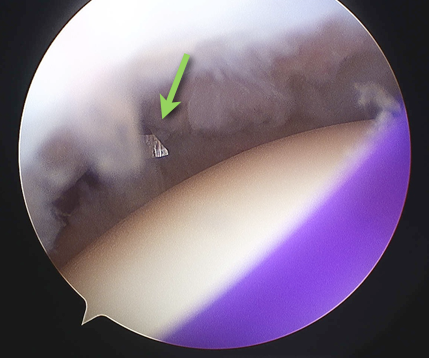
Figure 4. SEM images of the core layer, the intermediate layer and the superficial articular layer (Hill et al. 2008).
The role of the labrum
The exact functional role of the labrum is still undefined. It has been theorised to function as a chock-block, increasing the glenoid depth and resisting translation (Matsen, III et al. 1991; Lippitt and Matsen 1993). Concavity compression is an extension of this postulate, whereby the humeral head is compressed into the cavity of the glenoid by the rotator cuff musculature, further stabilising the shoulder. This mechanism has been calculated to increase stability by 10-20% (Lippitt and Matsen 1993; Fehringer et al. 2003) with an intact labrum and may be due to its role in centralising the head within the glenoid (Fehringer et al. 2003). The labrum also helps to maintain a negative intra-articular pressure within the joint, which itself confers stability (Habermeyer et al. 1992); the magnitude of this effect has not been quantified. Loss of the anterior-inferior labrum in cadaveric specimens has been shown to decrease the contact area of the articular surface of the glenohumeral joint and increase the mean contact pressure (Greis et al. 2002).
Huber and Putz (Huber and Putz 1997) proposed the periarticular fibre system of the shoulder, which includes the GHL's (glenohumeral ligaments), the labrum, the long head of triceps and the long head of biceps forming a complete ring of ligamentous and tendinous tissue that runs through the labrum in continuity. Their study of 42 cadaveric shoulders demonstrated the superior GHL inserting into the anterior aspect of the biceps anchor of the superior labrum. The biceps tendon then runs into the posterior labrum as far as the posterioinferior quadrant. In the inferior area fibres from the long tendon of triceps run back into the labrum and this tendon often (38%) has fibres running into the anterior part of the inferior labrum. This is the insertional point of the inferior GHL, which they then describe joining the superior GHL by a 'band of tissue' spanning the anterosuperior quadrant and completing the ring. In agreement with this, other authors have also demonstrated a connecting band from the inferior GHL of the anterior inferior labrum to the biceps tendon (Tuoheti et al. 2005). This periarticular fibre system is proposed as a tension brace to buttress against the humeral head and spread the pressure over a wide area. They do not comment on the presence of the middle GHL at all.

Figure 5. Original diagrammatic presentation of the periarticular fibre system taken from Huber and Putz (1997).
Interestingly the Buford complex (Williams et al. 1994) may actually be the band described by Huber and Putz from the IGHL to the SGHL. Even a 'normal' looking MGHL may be the same band and highlights the difficulty in interpretation of the anatomy.  top
top
Biomechanical studies
Simple shear tests around the circumference of the glenoid, which involved an increasing force applied to the labrum in a plane parallel to the surface of the glenoid (Reeves 1968; Hara et al. 1996) concluded that the 4 'o'clock position was found to be the weakest and the 7 'o'clock position the strongest. The interface of failure being between the labrum and the glenoid rather than the labrum itself.
Studies have been devised to try and recreate which loading scenarios may lead to a SLAP lesion. There is disagreement as to whether pure traction on the biceps tendon can create type II SLAP lesions, with some authors claiming it can (Bey et al. 1998) and others claiming it cannot (Costa et al. 2006).
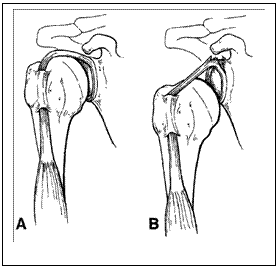
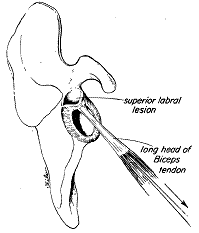
Figure 6. A and B) Uniaxial loading of biceps tendon leading to SLAP lesion in the neutral position (Bey et al. 1998) and C) during the late cocking phase of throwing (Rodosky et al. 1994).
Other cadaveric studies have concentrated on the scenario during throwing with 25% of specimens creating a SLAP type II lesion, of which 80% are created in late cocking position (Kuhn et al. 2003). Another simulation of the late cocking phase of throwing with further external rotation of the humerus 20% beyond its normal maximal external rotation also created SLAP II lesions (Mihata et al. 2008).
Biomechanical testing of the labrum itself has been performed with compressive testing on embalmed specimens (Carey et al. 2000). The elastic modulus was found to be greater in the superior part of the labrum compared with the inferior sections and they also noted that the dominant side, of each shoulder pair, consistently had a thicker labrum.
The compressive and tensile properties of the labrum have also been studied in fresh frozen specimens (Smith et al. 2008; Smith et al. 2009). These studies demonstrated that the tensile elastic modulus was 22.8 MPa, which is much closer to articular cartilage (2-20 MPa) than to the meniscus (170 MPa). Also the anterosuperior portion had a lower tensile elastic modulus than the inferior portion. Conversely the compressive modulus was higher in the anterosuperior portion.  top
top
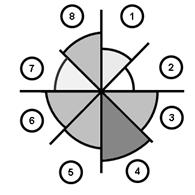
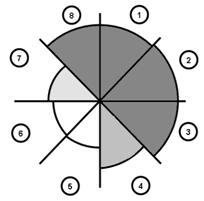
Figure 7. Greyscale grading of the a) tensile and b) compressive elastic modulus of the glenoid labrum (Smith et al. 2008; Smith et al. 2009).
Clinical Lesions
Although there are many hypotheses on the function of the labrum, there is agreement that it aids in stability of the shoulder. The shoulder is the most mobile joint in the body, but is also the most commonly dislocated joint in the body, with an incidence reported at 1-1.7% of the adult population (McFarland et al. 1996) (Hovelius 1982). The prevalence of recurrent shoulder dislocation under the age of 21 is 19.7 in 10,000 for males and 5 in 10,000 for females (Milgrom et al. 1998). There has been multiple pathological lesions of the capsular-labral complex described associated with shoulder dislocation or recurrent subluxation.
The mostly commonly associated with an anterior dislocation is the Bankart lesion. This was originally described as a detached segment of the anterior-inferior labrum with its attached inferior glenohumeral ligament complex (Bankart 1923). It has been found in 39% of first time dislocators as seen on MR arthrogram (Antonio et al. 2007), 75% as seen on plain MRI (Widjaja et al. 2006) and as high as 83- 97% in the young patient seen at arthroscopy (Taylor and Arciero 1997; Yiannakopoulos et al. 2007).

Figure 8. Position of rupture in Bankart lesion (Miles and Tasto 2004)
The failure point in a Bankart lesion is at the interface between the labrum and the glenoid rim, or in a bony Bankart lesion failure is within the glenoid bone itself.
An ALPSA (Anterior labrocapsular periosteal sleeve avulsion) lesion differs from a Bankart lesion in that the periosteum of the anterior scapular does not rupture (Neviaser 1993). A variant of the ALPSA affecting the superior part of the anterior labrum has also been described (Atay et al. 2002). Neither or these lesions compromise the labrum itself.
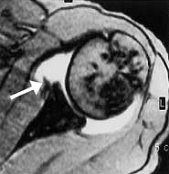
Figure 9. A T2 weighted MRI imaging demonstrating an ALPSA lesion (Atay et al. 2002).
The POLPSA (posterior labrocapsular periosteal sleeve avulsion) lesion has been described predominantly in American football players with posterior shoulder instability (Mair et al. 1998; Yu et al. 2002; Escobedo et al. 2007). Although the original description was in a patient with a locked posterior dislocation (Simons et al. 1998). Again the point of failure is between the labrum and the glenoid.
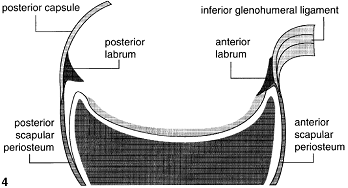
Figure 10. The POLPSA lesion demonstrating the point of injury between the labrum and glenoid (Simons et al. 1998).
Kim's lesion appears to be a partial POLPSA with incomplete tearing between the posterioinferior labrum and the glenoid articular cartilage associated with multi or unidirectional instability (Kim et al. 2004).
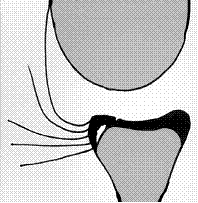
Figure 11. Original drawing of Kim's lesion (Kim et al. 2004).
Although all of the above lesions do affect stability of the shoulder, none are a direct injury to the labrum itself. The SLAP (superior labrum anterior and posterior) lesion is a tear of the labrum itself. The original terminology and classification was by Snyder et al. in 1990.
This described four lesions:
- Type I -fraying and degenerative of the superior labrum, but peripheral labral edge still attached to underlying glenoid
- Type II - detachment of the superior labrum and biceps tendon from underlying glenoid. Also has fraying and degeneration as Type I.
- Type III - bucket handle tear of superior labrum. Remaining labral tissue anchored to glenoid rim
- Type IV - extension of displaced bucket handle tear into biceps tendon
Lesions have also been described similar to a Type II SLAP, but with associated detachment of articular cartilage and exposure of underlying cartilage (Choi and Kim 2004).
Maffet et al. (1995) extended the classification to add a further three types.
- Type V - a Bankart lesion that continues superiorly to include separation of the biceps tendon.
- Type VI - biceps separation with an unstable flap tear of the labrum.
- Type VII - superior labral biceps tendon separation that extends anteriorly beneath the middle GHL.
In 1998 Morgan et al. sub-divided Type II lesion into three groups depending on the anatomical location of the lesion.
More recently Nord and Ryu have added a further three types of lesions to the classification (Powell et al. 2004).
- Type VIII - a SLAP extension along the posterior glenoid labrum as far as the 6 'o'clock position.
- Type IX - a pan-labral SLAP injury extending the entire circumference of the glenoid labrum.
- Type X - a superior labral tear associated with a posterior-inferior labral tear.
The classifications for SLAP lesions are arbitrary and can really be divided into three broad groups:
- Fraying / degeneration of the labrum (type I)
- Detachment between the labrum and the glenoid (type II, V, VII, VIII, IX and X)
- Mid-substance tears (type III and IV)
Type VI appears to be a combination of a detachment and mid-substance tear.
SLAP tears in general have been associated with shoulder dislocations seen on MR arthrogram in 14% of patients (majority type II) (Antonio et al. 2007)and at arthroscopy in 7-20% of patients (Hintermann and Gachter 1995; Taylor and Arciero 1997; Werner et al. 2004; Yiannakopoulos et al. 2007). When seen at arthroscopy the majority (up to 81%) of SLAP lesions are Type II (Snyder et al. 1990; Snyder et al. 1995; Maffet et al. 1995; Kampa and Clasper 2005; Yiannakopoulos et al. 2007; Park et al. 2008). Although other authors have described Type I as the most common lesion (74%) (Kim et al. 2003), especially in atraumatic dislocations (Werner et al. 2004). Only type I and II SLAP lesions were found in first time dislocators, whereas all types including the more severe type III and IV were found in recurrent dislocators (Yiannakopoulos et al. 2007).
When present the frequency of the type of SLAP lesion found varied in the literature. Amalgamating results from multiple studies would give a combined incidence of 36% type I, 53% type II, 6% type III and 5% type IV (Snyder et al. 1995; Kim et al. 2003; Kampa and Clasper 2005). However, there appears to be a difference in reporting of type I lesions between studies and this poor inter-observer and intra-observer variability of the Snyder SLAP classification has been noted in the literature (Gobezie et al. 2008). The predominance of type II lesions for SLAP lesions undergoing repair has been shown (88% type II, 4% type III and 8% type IV) (Park et al. 2008).
Anatomical variations that have previously been discussed may play a role in the susceptibility of a patient to SLAP tears. Certainly a Buford complex has been noted to increase the rate of SLAP injuries (Nam and Snyder 2003; Bents and Skeete 2005). From 235 arthroscopic assessments 6 patients were found to have Buford complexes, of which 5 (83%) had an associated SLAP lesion (predominantly type II).  top
top
Summary
In summary the literature shows form both embryological and anatomical studies that the anterosuperior region of the labrum is different to the remainder of its circumference. The histology suggests that it is essentially a structure designed to resist circumferential tensile loading. The superior region of the labrum appears to the most prone to intra-substance tears. Multiple theories for the mechanism of tearing have been proposed, but essentially the two schools of thought are that the labrum is either pinched or compressed with internal impingement, or the labrum is pulled directly or twisted off by the biceps anchor. Therefore the mechanism of failure may be by compression, direct tension, or torsional tension. The biomechanical studies in the literature have confirmed that all three types of mechanism can create a SLAP lesion. It is unlikely that type II lesions will progress to type III or IV as they have different points of failure. Therefore the mechanisms of injury for each type of SLAP lesion may be different.
Reference List
- Aboul-Mahasen, L. M. and Sadek, S. A. (2002). Developmental morphological and histological studies on structures of the human fetal shoulder joint. Cells Tissues.Organs 170, 1-20.
- Anetzberger, H. and Putz, R. (1996). The scapula: principles of construction and stress. Acta Anat.(Basel) 156, 70-80.
- Antonio, G. E., Griffith, J. F., Yu, A. B., Yung, P. S., Chan, K. M., and Ahuja, A. T. (2007). First-time shoulder dislocation: High prevalence of labral injury and age-related differences revealed by MR arthrography. J Magn Reson.Imaging 26, 983-991.
- Atay, O. A., Aydingoz, U., Doral, M. N., and Leblebicioglu, G. (2002). Anterior labroligamentous periosteal sleeve avulsion lesion at the superior glenoid labrum. Knee.Surg.Sports Traumatol.Arthrosc. 10, 122-125.
- Bankart, A. S. B. (1923). Recurrent or habitual dislocation of the shoulder joint. British Medical Journal 2, 1132-1133.
- Bents, R. T. and Skeete, K. D. (2005). The correlation of the Buford complex and SLAP lesions. J Shoulder.Elbow.Surg 14, 565-569.
- Bey, M. J., Elders, G. J., Huston, L. J., Kuhn, J. E., Blasier, R. B., and Soslowsky, L. J. (1998). The mechanism of creation of superior labrum, anterior, and posterior lesions in a dynamic biomechanical model of the shoulder: the role of inferior subluxation. J.Shoulder.Elbow.Surg. 7, 397-401.
- Boileau, P. and Walch, G. (1997). The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J.Bone Joint Surg.Br. 79, 857-865.
- Buford, D., Snyder, S. J., Veneziani, S., and Wuh, H. C. K (1992). The Buford complex -The loose anterior superior labrum/middle glenohumeral ligament complex: an anatomic variant. Arthroscopy 8, 402.
- Carey, J., Small, C. F., and Pichora, D. R. (2000). In situ compressive properties of the glenoid labrum. J.Biomed.Mater.Res. 51, 711-716.
- Choi, N. H. and Kim, S. J. (2004). Avulsion of the superior labrum. Arthroscopy 20, 872-874.
- Clavert, Philippe, Kempf, Jean Francois, Wolfram-Gabel, Rennie, and Kahn, Jean Luc (2005). Are there age induced morphologic variations of the superior glenoid labrum? About 100 shoulder arthroscopies. Surgical and Radiologic Anatomy 27, 385-388.
- Cooper, D. E., Arnoczky, S. P., O'Brien, S. J., Warren, R. F., DiCarlo, E., and Allen, A. A. (1992). Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J.Bone Joint Surg.Am. 74, 46-52.
- Costa, Ado S., Leite, J. A., Melo, F. E., and Guimaraes, S. B. (2006). Biomechanical properties of the biceps-labral complex submitted to mechanical stress. Acta Cir.Bras. 21, 214-218.
- Davidson, Philip A. and Rivenburgh, Dennis W. (2004). Mobile Superior Glenoid Labrum: A Normal Variant or Pathologic Condition? American Journal of Sports Medicine 32, 962-966.
- De Palma, A. F., Callery, C., and Bennett, G. A (1949). Variational anatomy and degenerative lesions of the shoulder joint. AAOS Instructional Course Lectures 6, 255-281.
- Demondion, X., Maynou, C., Van, Cortenbosch B., Klein, K., Leroy, X., and Mestdagh, H. (2001). [Relationship between the tendon of the long head of the biceps brachii muscle and the glenoid labrum]. Morphologie. 85, 5-8.
- Escobedo, E. M., Richardson, M. L., Schulz, Y. B., Hunter, J. C., Green, J. R., III, and Messick, K. J. (2007). Increased risk of posterior glenoid labrum tears in football players. AJR Am.J Roentgenol. 188, 193-197.
- Fealy, S., Rodeo, S. A., Dicarlo, E. F., and O'Brien, S. J. (2000). The developmental anatomy of the neonatal glenohumeral joint. J.Shoulder.Elbow.Surg. 9, 217-222.
- Fehringer, E. V., Schmidt, G. R., Boorman, R. S., Churchill, S., Smith, K. L., Norman, A. G., Sidles, J. A., and Matsen, F. A., III (2003). The anteroinferior labrum helps center the humeral head on the glenoid. J.Shoulder.Elbow.Surg. 12, 53-58.
- Gardner, E. and Gray, D. J. (1953). Prenatal development of the human shoulder and acromioclavicular joints. Am.J Anat. 92, 219-276.
- Gobezie, R., Zurakowski, D., Lavery, K., Millett, P. J., Cole, B. J., and Warner, J. J. (2008). Analysis of Interobserver and Intraobserver Variability in the Diagnosis and Treatment of SLAP Tears Using the Snyder Classification. Am.J Sports Med.
- Greis, P. E., Scuderi, M. G., Mohr, A., Bachus, K. N., and Burks, R. T. (2002). Glenohumeral articular contact areas and pressures following labral and osseous injury to the anteroinferior quadrant of the glenoid. J Shoulder Elbow Surg 11, 442-451.
- Guanche, C. A., Noble, J., Solomonow, M., and Wink, C. S. (1999). Periarticular neural elements in the shoulder joint. Orthopedics 22, 615-617.
- Habermeyer, P., Schuller, U., and Wiedemann, E. (1992). The intra-articular pressure of the shoulder: an experimental study on the role of the glenoid labrum in stabilizing the joint. Arthroscopy 8, 166-172.
- Haines, R. W. (1947a). The development of joints. J Anat. 81, 33-55.
- Haines, R. W. (1947b). The development of joints. J Anat. 81, 33-55.
- Hara, H., Ito, N., and Iwasaki, K. (1996). Strength of the glenoid labrum and adjacent shoulder capsule. J Shoulder Elbow Surg 5, 263-268.
- Harzmann, H. C., Burkart, A., Wortler, K., Vaitl, T., and Imhoff, A. B. (2003). Normal anatomical variants of the superior labrum biceps tendon anchor complex. Anatomical and magnetic resonance findings. Orthopade 32, 586-594.
- Hertel, R., Knothe, U., and Ballmer, F. T. (2002). Geometry of the proximal humerus and implications for prosthetic design. J.Shoulder.Elbow.Surg. 11, 331-338.
- Hertz, H., Weinstabl, R., Grundschober, F., and Orthner, E. (1986). [Macroscopic and microscopic anatomy of the shoulder joint and the limbus glenoidalis]. Acta Anat.(Basel) 125, 96-100.
- Hill, A. M., Hoerning, E. J., Brook, K., Smith, C. D., Moss, J., Ryder, T., Wallace, A. L., and Bull, A. M. (2008). Collagenous microstructure of the glenoid labrum and biceps anchor. J Anat. 212, 853-862.
- Hintermann, B. and Gachter, A. (1995). Arthroscopic findings after shoulder dislocation. Am.J Sports Med. 23, 545-551.
- Hovelius, L. (1982). Incidence of shoulder dislocation in Sweden. Clin.Orthop.Relat Res. 127-131.
- Howell, S. M. and Galinat, B. J. (1989). The glenoid-labral socket. A constrained articular surface. Clin.Orthop.Relat Res. 122-125.
- Huber, W. P. and Putz, R. V. (1997). Periarticular fiber system of the shoulder joint. Arthroscopy 13, 680-691.
- Ide, J., Maeda, S., and Takagi, K. (2004). Normal variations of the glenohumeral ligament complex: an anatomic study for arthroscopic Bankart repair. Arthroscopy 20, 164-168.
- Kampa, R. J. and Clasper, J. (2005). Incidence of SLAP lesions in a military population. J R.Army Med.Corps 151, 171-175.
- Kim, S. H., Ha, K. I., Yoo, J. C., and Noh, K. C. (2004). Kim's lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy 20, 712-720.
- Kim, T. K., Queale, W. S., Cosgarea, A. J., and McFarland, E. G. (2003). Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 85-A, 66-71.
- Kuhn, J. E., Lindholm, S. R., Huston, L. J., Soslowsky, L. J., and Blasier, R. B. (2003). Failure of the biceps superior labral complex: a cadaveric biomechanical investigation comparing the late cocking and early deceleration positions of throwing. Arthroscopy 19, 373-379.
- Lippitt, S. and Matsen, F. (1993). Mechanisms of glenohumeral joint stability. Clin.Orthop.Relat Res. 20-28.
- Machner, A., Wissel, H., Heitmann, D., and Pap, G. (1998). [Changes in proprioceptive capacities of the shoulder joint in ventral shoulder instability. A comparative study before and after arthroscopic labrum refixation]. Sportverletz.Sportschaden 12, 138-141.
- Maffet, M. W., Gartsman, G. M., and Moseley, B. (1995). Superior labrum-biceps tendon complex lesions of the shoulder. Am.J.Sports Med. 23, 93-98.
- Mair, S. D., Zarzour, R. H., and Speer, K. P. (1998). Posterior labral injury in contact athletes. Am.J Sports Med. 26, 753-758.
- Matsen, F. A., III, Harryman, D. T., and Sidles, J. A. (1991). Mechanics of glenohumeral instability. Clin Sports Med 10, 783-788.
- McFarland, E. G., Torpey, B. M., and Curl, L. A. (1996). Evaluation of shoulder laxity. Sports Med 22, 264-272.
- McPherson, E. J., Friedman, R. J., An, Y. H., Chokesi, R., and Dooley, R. L. (1997). Anthropometric study of normal glenohumeral relationships. J.Shoulder.Elbow.Surg. 6, 105-112.
- Mihata, T., McGarry, M. H., Tibone, J. E., Fitzpatrick, M. J., Kinoshita, M., and Lee, T. Q. (2008). Biomechanical Assessment of Type II Superior Labral Anterior-Posterior (SLAP) Lesions Associated With Anterior Shoulder Capsular Laxity as Seen in Throwers: A Cadaveric Study. Am.J Sports Med. doi:10.1177/0363546508315198 (available at ajs.sagepub.com).
- Miles, J. W and Tasto, J. P. (2004). Arthroscopic Bankart repair of anterior shoulder instabilityin the athelete. Oper Tech Sports Med 12, 126-134.
- Milgrom, C., Mann, G., and Finestone, A. (1998). A prevalence study of recurrent shoulder dislocations in young adults. J Shoulder.Elbow.Surg 7, 621-624.
- Morgan, C. D., Burkhart, S. S., Palmeri, M., and Gillespie, M. (1998). Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy 14, 553-565.
- Moseley, H. F. and Overgaard, B. (1962). The anterior capsular mechanism in recurrent anterior dislocation of the shoulder. Journal of Bone and Joint Surgery - British Volume 44-B, 913-927.
- Nam, E. K. and Snyder, S. J. (2003). The diagnosis and treatment of superior labrum, anterior and posterior (SLAP) lesions. Am.J Sports Med. 31, 798-810.
- Neviaser, T. J. (1993). The anterior labroligamentous periosteal sleeve avulsion lesion: a cause of anterior instability of the shoulder. Arthroscopy 9, 17-21.
- Nishida, K., Hashizume, H., Toda, K., and Inoue, H. (1996). Histologic and scanning electron microscopic study of the glenoid labrum. J Shoulder Elbow Surg 5, 132-138.
- Pal, G. P., Bhatt, R. H., and Patel, V. S. (1991). Relationship between the tendon of the long head of biceps brachii and the glenoidal labrum in humans. Anat.Rec. 229, 278-280.
- Park, J. H., Lee, Y. S., Wang, J. H., Noh, H. K., and Kim, J. G. (2008). Outcome of the isolated SLAP lesions and analysis of the results according to the injury mechanisms. Knee.Surg Sports Traumatol.Arthrosc. 16, 511-515.
- Pfahler, M., Haraida, S., Schulz, C., Anetzberger, H., Refior, H. J., Bauer, G. S., and Bigliani, L. U. (2003). Age-related changes of the glenoid labrum in normal shoulders. J.Shoulder.Elbow.Surg. 12, 40-52.
- Pouliart, N., Somers, K., Eid, S., and Gagey, O. (2007). Variations in the superior capsuloligamentous complex and description of a new ligament. J Shoulder.Elbow.Surg 16, 821-836.
- Powell, S. E., Nord, K. D., and Ryu, R. K. N (2004). The diagnosis, classification and treatment of SLAP lesions. Oper Tech Sports Med 12, 99-110.
- Prodromos, C. C., Ferry, J. A., Schiller, A. L., and Zarins, B. (1990). Histological studies of the glenoid labrum from fetal life to old age. Journal of Bone and Joint Surgery 72, 1344-1348.
- Rao, A. G., Kim, T. K., Chronopoulos, E., and McFarland, E. G. (2003). Anatomical variants in the anterosuperior aspect of the glenoid labrum: a statistical analysis of seventy-three cases. J.Bone Joint Surg.Am. 85-A, 653-659.
- Reeves, B (1968). Experiments on the tensile strength of the anterior capsular structures of the shoulder in man. The Journal of Bone and Joint Surgery (British) 50, 858-868.
- Rodosky, M. W., Harner, C. D., and Fu, F. H. (1994). The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am.J.Sports Med. 22, 121-130.
- Simons, P., Joekes, E., Nelissen, R. G., and Bloem, J. L. (1998). Posterior labrocapsular periosteal sleeve avulsion complicating locked posterior shoulder dislocation. Skeletal Radiol. 27, 588-590.
- Smith, C. D., Masouros, S. D., Hill, A. M., Wallace, A. L., Amis, A. A., and Bull, A. M. (2009). The compressive behavior of the human glenoid labrum may explain the common patterns of SLAP lesions. Arthroscopy 25, 504-509.
- Smith, C. D., Masouros, S. D., Hill, A. M., Wallace, A. L., Amis, A. A., and Bull, A. M. J. (2008). Tensile properties of the human glenoid labrum. Journal of Anatomy 212, 49-54.
- Snyder, S. J., Banas, M. P., and Karzel, R. P. (1995). An analysis of 140 injuries to the superior glenoid labrum. J.Shoulder.Elbow.Surg. 4, 243-248.
- Snyder, S. J., Karzel, R. P., Del, Pizzo W., Ferkel, R. D., and Friedman, M. J. (1990). SLAP lesions of the shoulder. Arthroscopy 6, 274-279.
- Soslowsky, L. J., Flatow, E. L., Bigliani, L. U., and Mow, V. C. (1992). Articular geometry of the glenohumeral joint. Clin.Orthop.Relat Res. 181-190.
- Steinbeck, J., Liljenqvist, U., and Jerosch, J. (1998). The anatomy of the glenohumeral ligamentous complex and its contribution to anterior shoulder stability. J.Shoulder.Elbow.Surg. 7, 122-126.
- Taylor, D. C. and Arciero, R. A. (1997). Pathologic changes associated with shoulder dislocations. Arthroscopic and physical examination findings in first-time, traumatic anterior dislocations. Am.J.Sports Med. 25, 306-311.
- Tena-Arregui, J., Barrio-Asensio, C., Puerta-Fonolla, J., and Murillo-Gonzalez, J. (2005). Arthroscopic study of the shoulder joint in fetuses. Arthroscopy 21, 1114-1119.
- Tuoheti, Y., Itoi, E., Minagawa, H., Yamamoto, N., Saito, H., Seki, N., Okada, K., Shimada, Y., and Abe, H. (2005). Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy 21, 1242-1249.
- Vangsness, C. T., Jr., Ennis, M., Taylor, J. G., and Atkinson, R. (1995). Neural anatomy of the glenohumeral ligaments, labrum, and subacromial bursa. Arthroscopy 11, 180-184.
- Vangsness, C. T., Jr., Jorgenson, S. S., Watson, T., and Johnson, D. L. (1994). The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. The Journal of Bone and Joint Surgery (Proceedings) 76, 951-954.
- Waldt, S., Metz, S., Burkart, A., Mueller, D., Bruegel, M., Rummeny, E. J., and Woertler, K. (2006). Variants of the superior labrum and labro-bicipital complex: a comparative study of shoulder specimens using MR arthrography, multi-slice CT arthrography and anatomical dissection. Eur.Radiol. 16, 451-458.
- Werner, A. W., Lichtenberg, S., Schmitz, H., Nikolic, A., and Habermeyer, P. (2004). Arthroscopic findings in atraumatic shoulder instability. Arthroscopy 20, 268-272.
- Widjaja, A. B., Tran, A., Bailey, M., and Proper, S. (2006). Correlation between Bankart and Hill-Sachs lesions in anterior shoulder dislocation. ANZ.J.Surg. 76, 436-438.
- Williams, M. M., Snyder, S. J., and Buford, D., Jr. (1994). The Buford complex--the "cord-like" middle glenohumeral ligament and absent anterosuperior labrum complex: a normal anatomic capsulolabral variant. Arthroscopy 10, 241-247.
- Yiannakopoulos, C. K., Mataragas, E., and Antonogiannakis, E. (2007). A comparison of the spectrum of intra-articular lesions in acute and chronic anterior shoulder instability. Arthroscopy 23, 985-990.
- Yu, J. S., Ashman, C. J., and Jones, G. (2002). The POLPSA lesion: MR imaging findings with arthroscopic correlation in patients with posterior instability. Skeletal Radiol. 31, 396-399.


