Night pain reduction following arthroscopic subacromial decompression
References: BESS 2005
One reason patients may seek surgical intervention for shoulder pain is when their sleep pattern is significantly disturbed. The aim of this study was to analyse the reduction in patients night pain, secondly to ascertain if patients night pain relief related to operative findings and thirdly to correlate results with the night pain components of the Constant and Oxford scores.
Over a one year period ASD was performed on 38 male and 42 female patients suffering from chronic shoulder impingement. All patients completed a 100mm visual analogue night pain scale (VAS), Constant and Oxford scores at 3, 6, 12 and 24 weeks postoperatively. Comparisons between night pain scores were performed at each time scale using paired samples tests. Degree of impingement was based on the Copeland/Levy classification and comparisons made with mean night pain scores at 24 weeks. Correlations were made between the VAS and the night pain components of the Constant and Oxford scores also at each time scale.
As determined by the VAS all patients pre operatively experienced night pain, some considerable.
By three weeks the average night pain significantly reduced from 60.31 (95% CI, 55.65-64.99) to 46.19 (95% CI, 40.52-51.86) [a reduction of 15%].
By 24 weeks, 77 out of 80 patients (96%) had reduced their night pain levels with 20 patients (25%) having no night pain at all.
In 51 out of 80 patients (64%), their night pain was reduced by more than 50% and in 36 patients (45%) by more than 75%.
All classifications of patients improved their night pain status, with no significant differences regarding operative findings. The least improvement occurred in the A0 B0 category.
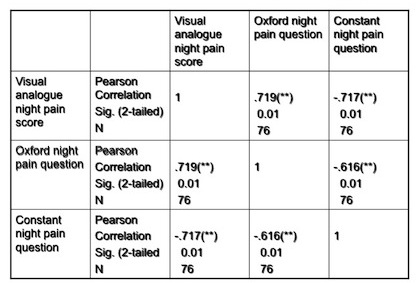
The VAS correlated significantly (p<0.01) with the Constant night pain (-.717) and Oxford (.719) at 24 weeks and at every other stage.
ASD is an effective procedure for reducing patients levels of night pain.
Figure 1: After 3 weeks, 55 out of 80 patients had reduced their night pain levels compared with preoperatively. After 6 weeks 62 patients had improved. After 12 weeks 68 patients had improved. And after 24 weeks 77 out of 80 patient’s night pain levels had improved.
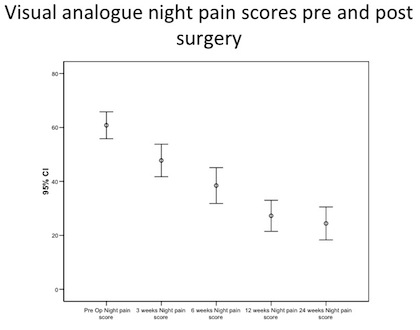
Figure 2 represents the percentage of patients who experienced more than 50% reduction in night pain at each time scale.
After 3 weeks 21% of patients who experienced more than 50% reduction in night pain at each time scale. After 6 weeks this was 42%, after 12 weeks 60% and after 24 weeks 64%.
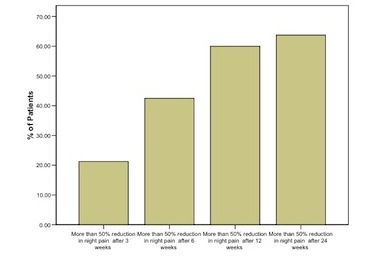
Figure 3: the percentage of patients experiencing more than 75% reduction in night pain at each time scale.
After 3 weeks 9% of patients experiencing more than 75% reduction in night pain at each time scale, after 6 weeks 23%, after 12 weeks 35% and after 24 weeks 45%. By this stage 20 patients had no night pain at all.
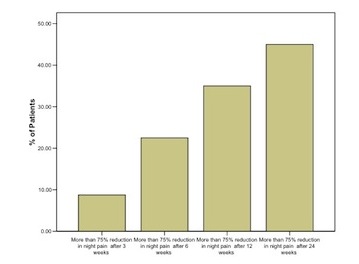
Figure 4: Relationship between degree of impingement and night pain reduction. In this graph the purple bars demonstrate preoperative night pain levels and the green bars the degree of night pain remaining at 24 weeks. The degree of impingement, based on the Copeland/Levy classification, is represented along the bottom axis. It can be seen that all classifications of impingement improve. The one with the least reduction in night pain is the A0B0 category.
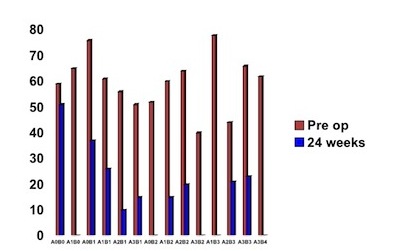
Figure 5: Results of correlations between pain measurements 24 weeks post surgery. This table demonstrates the correlation between the VAS and the night pain questions of the Constant score and Oxford score at 24 weeks. The VAS correlated significantly with the Constant night pain and Oxford night pain questions at 24 weeks and also at every other time scale.